
Aging and Stress within Medical Coatings(by J.J.G. Bos)
Causes of Stress and Strain in Coatings Detection of Stress and Strain in Coatings Aging Mechanisms of Medical Coatings and Medical Devices Accelerated Aging of Medical Coatings and Medical Devices Effects of Stress and Strain Forces in Coatings
Forces in the plane of the coating generally also induce pull-forces across the interface between coating and device. These forces may be so high that the coating dislodges from the device. Ideally, interfacial attraction-forces are significantly higher than the pull-forces caused by the bulk of the coating, so that no or little damage is done. The forces in the coating may give rise to cracking of the coating, which in itself causes localized strain-relief. But the question is what happened prior to cracking (dislodging?), and is the strain-relief sufficient to prevent future interfacial damage?
return to top... Causes of Stress and Strain in CoatingsThere are several causes of stress or strain forces in coatings, for example:
Shrinkage due to drying and setting generally happens shortly after application of the coating to the device, due to loss of solvent (drying). The thicker the coating, the greater the forces within the coating and the higher the chance of stress-cracking or dislodgement from the device. Stress-cracking is a force-relief mechanism. Temperature-induced stress and strain, as well as mechanically induced forces, generally affect the coating during transportation (e.g. aeroplane). These effects come on top of the forces that were already induced by drying and setting of the coating. Some coatings may give way much easier after transportation than before. Some hydrogel coatings adhere extremely well while dry, but eventually give way when re-hydrated upon use. Internal forces then already caused chain scission and interfacial breakdown during transportation and storage, but adhesion is maintained by the hydrophilic groups. However, these groups will start gathering water molecules around them upon re-hydration, thus letting go of each other: The coating weakens and may even completely fall apart. Depressing....
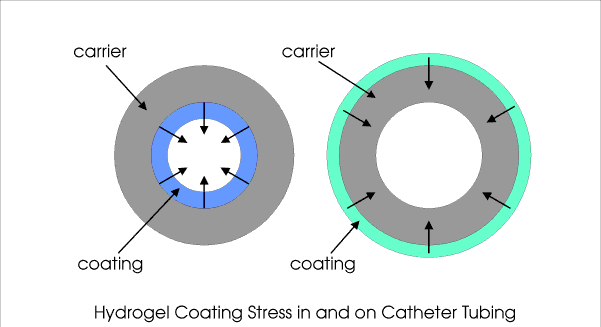 Illustration of stess-induced dislodgement of a hydrogel coating inside a catheter (picture left). return to top... Detection of Stress and Strain in CoatingsThere exists a variety of methods to detect stress and strain in coating layers. Indirect methods address investigation of the presence of cracks and local dislodgement. Direct methods involve force measurement. Examples are:
Adhesion of polyethylene lining can be evaluated with help of a simple peel test, utilizing a pull bench. Many coatings are hard to see and thus require some sort of staining method. Congo Red is often used for hydrogel coatings, but it tends to increase coating stress. Refer to the note on staining of hydrogels for illustration of this effect. Toluidine blue O is preferred for heparin-containing coatings. 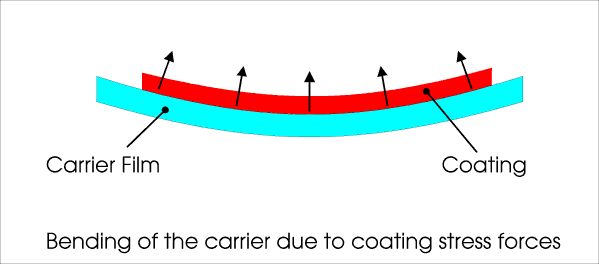 Illustration of strain-induced bending of a thin-film. Some resulting forces are shown. return to top... Aging Mechanisms of Medical Coatings and Medical DevicesBasic aging mechanisms are, for example:
The underlying causes were listed two paragraphs ago. Especially setting of the coating (molecular re-alignment) and temperature-induced stretching or shrinking of either the coating or the device are ongoing effects. They may slowly deteriorate the coating or its adhesion to the medical device. The rate of aging thus also depends on the storage method and environment. Storage near fluorescent tubes is not a good idea! Humidity is generally not appreciated by biodegradable coatings, whereas some hydrogel coatings remain compliant (read: tough) provided the air is not too dry. Places with lots of mechanical vibration or temperature fluctuation are hardly ever appreciated by medical devices, and in the end something must give. The aging effects are almost never reversible. return to top... Accelerated Aging of Medical Coatings and Medical DevicesThe basics of artificial acceleration of aging related to medical coatings and medical devices are found in doing everything you shouldn't do according to the previous paragraph. Think of running temperature and humidity cycles on the sterilized finished product. Repeated bending or elastic stetching of the coated product will also work. But how much should the coating be abused before one can tell that it passed the accelerated aging test? The answer to that is not always simple, since it strongly depends on the functionality of the coating and patient risk. The general approach must include aspects of the real-time life cycle of the product:
It is best to evaluate functionality and safety of the coating after each of these four steps. The first step is easiest to implement: Sterilization is just the real thing - no simulation. The second step can also be the real thing. I remember sending some samples around the world just for transportation testing! Simulation is not too hard either: Combine some temperature and humidity cycles with prolonged mechanical vibration - a few days should suffice. Storage simulation is where you want to gain time. One can think of accelerating the day and night cycle for temperature and add some accelerated seasonal humidity changes to it. Think of 1100 temperature cycles of 2.4 hours (1.2 hours high temperature, 1.2 hours low) combined with 3 humidity cycles of 1.2 months (0.6 months dry and 0.6 months humid) in order to accelerate the aging by factor 10. This whole cycle thus lasts 3.6 months, representing 3 years of real-time aging. Higher acceleration is possible, so long as the aspects of coating and device aging are well understood. Accelerated aging must completely relate to real-time aging and that may require some insight and testing for proof of principle. The weak aspects and definitely the risk apects of the coating form a good starting point. Note that the ISO-10993 series allows thermal acceleration, according to the Arrhenius or van 't Hoff rule for chemical reaction speeds. It is up to the engineer, however, to validate the accelerated aging process in the light of product-risk assessment. return to top... |