
Examples of Medical Device Development
Cetry helps medical device manufacturers and their suppliers by means of problem solving, process modelling, characterization of product properties, device and materials qualification, process and manufacturing validation, related regulatory issues, quality assurance, and design and build of specialized equipment. Here follow some examples of medical devices and device manufacturing techniques that were developed by Cetry.
List of Examples:
Related Pages:
Refer to examples of coating development in order to learn more about coatings developed for medical devices.
Refer to examples of equipment building in order to learn more about equipment built for medical device development and manufacturing processes.
Refer to examples of coating services in order to learn more about coating-related services for medical device development and manufacturing.
Refer to examples of QA-RA for quality- and regulatory-related topics regarding medical device development.
Unbraided F5 flush catheter for radiology
| • Client: | Start-up manufacturer of diagnostic catheters (radiology-cardiology) |
| • Subject: | Unbraided F5 flush catheter for radiology |
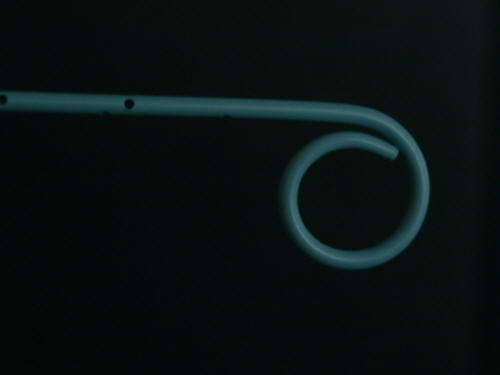
It was requested to deliver a F5 flush catheter for radiology. This type of catheter is used for inspection of the aorta. It had to (1) be able to withstand pressures up to 1050 psi (both dynamic and static), (2) have no body reinforcement by means of a metal wire braid, and (3) be able to punch through a small incision, thus allowing omittance of a catheter sheath introducer (CSI). The tip shape should be "pig-tail", with side holes correctly positioned for stable operation in the aorta, even at the highest possible injection flow of contrast media. Neither body nor tip were allowed to kink under intensive (but realistic) use. The catheter should be well visual under X-ray, and it should be very cheap. The latter put quite some restraints on the possibilities for manufacture. Design thus involved minimization of the number of components, maximization of the performance of the kind of tubing that was selected, and optimization of some assembly methods. The design was also made to look good. Luckily, the manufacturer had a wide variety of tubing at hand, so that design and manufacturing tests could start early on in the project. The catheter was intensively tested in the lab, prior to test-marketing. It performed up to expectation. The photograph at the top of this page shows a detail of the tip section of the catheter.
return to top...
Low-friction coaxial system
| • Client: | Major manufacturer of PTCA and neurological catheters |
| • Subject: | Low-friction coaxial catheter systems |
A design matrix was built to match catheters for coaxial application. Think of PTCA catheters going through a guiding catheter, or micro-catheters (for neurology) sliding over guide wires. This matrix allowed optimization of system performance rather than that of the individual parts. The latter works better for compatibility with (sometimes unknown) competitor devices, but system optimization usually leads to much better clinical performance. Drawback is that one has to convince the physician to buy and use the whole system, rather than use his or her favorite and trusted separate components/devices. However, this design program was meant to test the water. It was found that components of coaxial systems could easily be matched by proper choice of coatings and device flexibility. The results were obtained from in-vitro testing and modelling in combination with in-vivo evaluation. The spin-off was that one of the existing catheters was redesigned with a different coating and that other catheter types were followed by upgraded versions under different marketing names.
return to top...
Development of a new hub-to-tubing fusing technique
| • Client: | Start-up manufacturer of diagnostic catheters (radiology-cardiology) |
| • Subject: | Induction-heated hub-to-tubing fusion |
An induction-heating method was developed that is able to melt hub and catheter together. The catheter is put on a PTFE-coated iron rod and the hub is slid over the catheter. The parts must fit closely, of course. Heating of the rod by means of induction leads to melting and expansion of the catheter material. The hot catheter material will then fuse to the hub. This method is very delicate, since the components must be thermally matched (the right kind of melting points), but it is also very fast, reliable and clean. There is no adhesive involved, no solvents, no UV, and less chance of stress-crack related leakage. However, poor adjustment can lead to deformation of the hub. The results of the designed method were tested regarding pressure resistance, pull, sterilization, and aging. It proved to be a very neat manufacturing method.
return to top...